Thermal and Statistical Physics Assessment (TaSPA)
The Thermal and Statistical Physics Assessment (TaSPA) is designed to collect evidence of students’ abilities to do thermal and statistical physics and to provide actionable feedback that can inform course modifications to support student learning.
- Customize Assessments: Select specific learning objectives and content areas to focus on.
- Provide Regular Feedback: Generate detailed reports that help track student progress.
This tool empowers both instructors and students by identifying strengths and areas for improvement, ensuring a targeted and effective learning experience.
How it Works?
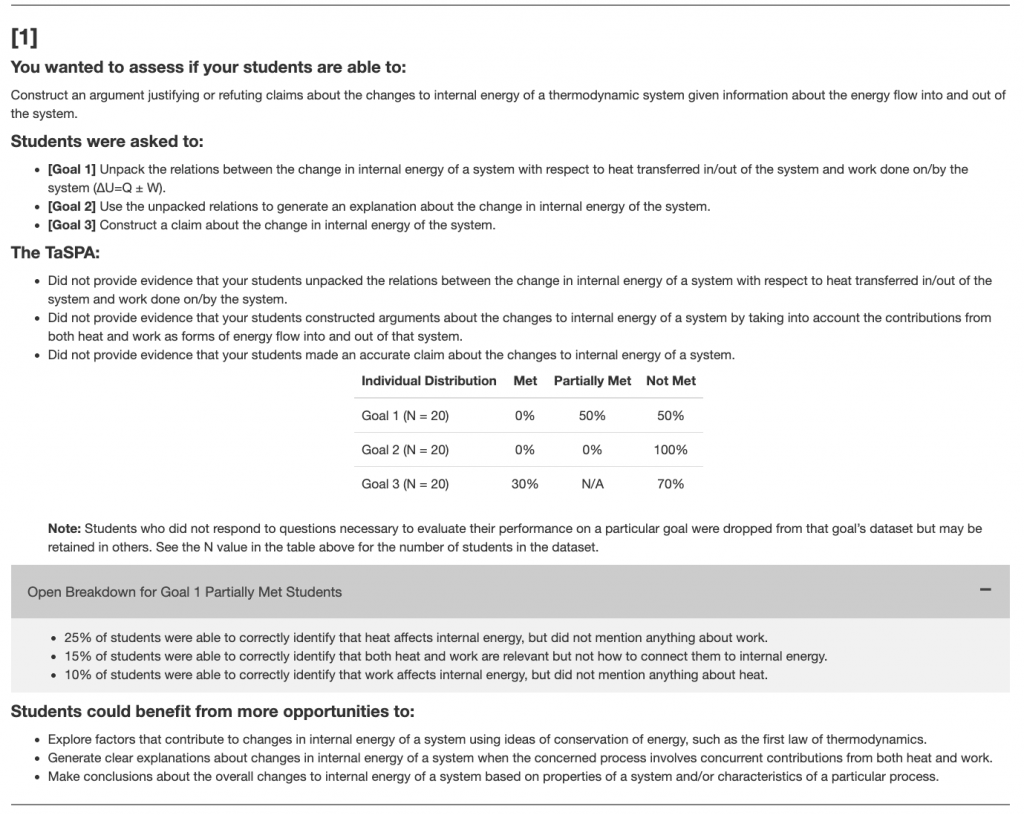
For each learning objective (LO), the TaSPA identifies the specific skills needed to solve the related task and characterizes students’ performance as Met (M), Partially-Met (P), or Not Met (N). A “Met” rating indicates that students provided both correct answers and sufficiently detailed reasoning, while “Partially-Met” indicates correct answers with incomplete reasoning. “Not Met” indicates that students did not provide correct answers or appropriate reasoning. For LOs that are Partially-Met or Not Met, the assessment offers suggestions that may help instructors adjust their courses.
Customizable: Instructors can choose specific learning objectives and administer assessments multiple times during a semester.
Time: This test usually takes 30 minutes.
Actionable Feedback: Students receive detailed feedback to track their progress and focus on areas requiring attention. The feedback highlights areas where students could benefit from more opportunities to deepen their understanding.
Continuous Monitoring: Instructors can monitor and support student growth throughout the course.
Benefits of Using Assessments
on LASSO
TaSPA covers 16 Learning Objectives
- Construct an argument justifying or refuting claims about the changes to internal energy of a thermodynamic system given information about the energy flow into and out of the system.
- Construct an argument justifying or refuting claims about the temperature of a system using information about changes in entropy and internal energy.
- Use mathematics to determine the number of microstates within a system to deduce the macroscopic quantity of entropy for that system and make a conclusion about the system.
- Analyze and interpret data about interacting systems to determine whether a thermodynamic process will happen spontaneously using the idea that entropy of the universe is maximized for spontaneous processes.
- Construct an argument justifying the most appropriate thermodynamic potential (e.g., Gibbs free energy, Helmholtz free energy, enthalpy) to be applied to a given context depending on system conditions (e.g., constant pressure, volume, or temperature).
- Generate an explanation about the mechanism by which the temperature does (or does not) change with heat flow into or out of a system informed by the process undergone and ambient conditions (e.g., pressure, temperature).
- Evaluate information in the form of ideas generated by students about the entropy of a system undergoing changes to its state by considering the number of microstates for a given macrostate.
- Use models to determine the number of microstates for a given macrostate and find the probability of a system being in that particular macrostate.
- Use a model to determine the probability of a system being in a particular state using the Boltzmann factor for that particular state and make relevant conclusions.
- Use representation(s) of the speed distribution of a gas to reason about its composition or properties.
- Use mathematics to calculate the temperature and internal energy and use them to predict macroscopic features of an Einstein solid given information about the entropy.
- Engage in argumentation regarding how (or if) a system’s partition function is impacted by contact with another system.
- Use mathematics to determine the heat capacity of argon and helium from the monatomic gas partition function and see if either gasses are usable in an experiment.
- Analyze and interpret data about the variation of pressure and volume inside an engine to determine the efficiency for a given amount of heat supplied over one cycle.
- Construct an explanation on how the physical dynamics of gasses determines how a scenario can be described with the ideal gas law.
- Use mathematics to determine the temperature of a black body from its radiated power.
Example Questions

Example TaSPA Report
The TaSPA report summarizes how students performed on the Thermal and Statistical Physics Assessment by presenting their results for the learning objectives (LOs) selected by the instructor:
- Each LO is broken down into specific goals, and student performance on these goals is labeled as Met, Partially-Met, or Not Met, based on the correctness and reasoning in their responses.
- The report provides guidance on where students may benefit from additional support and offers suggestions for how existing course activities could be adjusted to strengthen those skills.