Enthalpy and Entropy in Dissolution and Precipitation Inventory (E2DPI)
Purpose
The Enthalpy and Entropy in Dissolution and Precipitation Inventory (E2DPI) was developed to measure student understanding of the dissolution of ionic solutes, aqueous precipitation reactions, and the enthalpy and entropy changes that accompany these processes.
Population
The E2DPI is commonly administered in college chemistry courses.
Typical Performance
Below are the performance results from the initial instrument development for three populations: second-semester general chemistry (GC II), post-organic General Chemistry II (PO-GC II), and physical chemistry or biophysical chemistry (PC/BPC) at either a medium-sized midwestern university or a large, research intensive midwestern university.
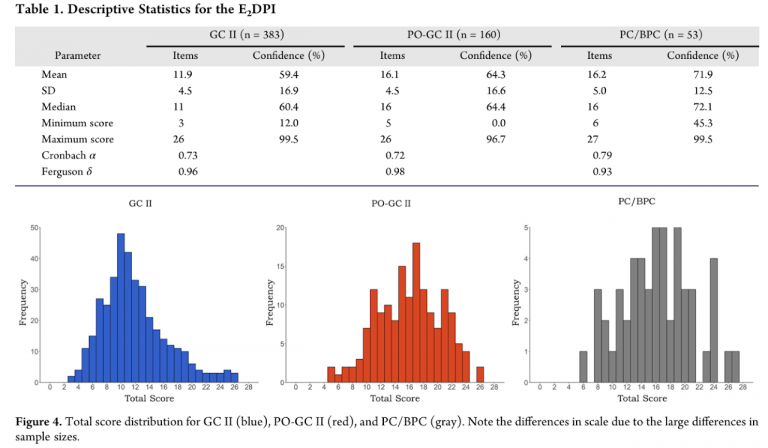
Validity
The E2DPI was originally developed in 2019. The development is detailed in
Abell, T. N., & Bretz, S. L. (2019). Development of the enthalpy and entropy in dissolution and precipitation inventory. Journal of Chemical Education, 96(9), 1804-1812.
Research
See the validation section for related articles.
Example LASSO Report
Please follow this link to our example report for concept inventories.

